+EFFICIENT +ECOLOGICAL +PROFITABLE
A polymer electrolyte fuel cell is a clean and efficient source of electrical power, which could, in the near future, be used in the automotive sector. However, the use of platinum and its alloys as catalysts in these new types of cells could prevent their widespread use, given the cost of these materials.
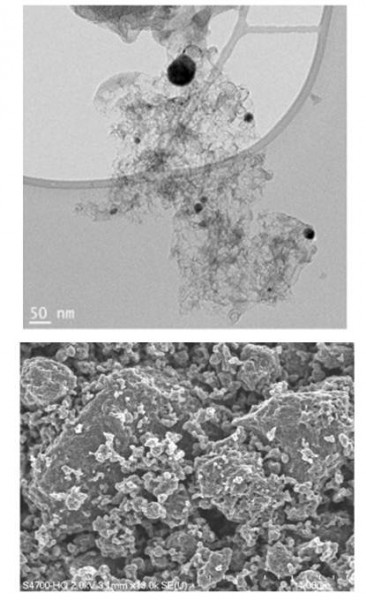
Work undertaken within the framework of this project demonstrates that a steel-based catalyst, a very common metal, could replace platinum. After having demonstrated during the course of an initial breakthough, that the catalytic activity of this type of catalyst rivalled that of platinum [1], a second significant breakthrough was recently obtained [2]. Once integrated in a fuel cell, this catalyst is capable of delivering electrical power comparable to that produced by platinum at a voltage that is of use in the field of automotive propulsion.
Before obtaining a fully marketable product, the last problem, which remains to be solved, relates to the long-term durability of the catalyst, which must be at least 5,000 hours in order to meet targets set by the US Department of Energy (DOE). Research is currently being conducted to find a solution to the current lack of durability.
REFERENCES
[1] M. Lefèvre, E. Proietti, F. Jaouen, J.-P. Dodelet, Science, 324, 71-74 (2009)
[2] E. Proietti, F. Jaouen, M. Lefèvre, N. Larouche, J. Tian, J. Herranz, J.-P. Dodelet, Nature Communications, 2, 416 (2011)
RESERCHERS
Prof. J.-P. Dodelet and M. Lefèvre (INRS)
COMPANY
Canétique Électrocatalyse
IRDQ’S CONTRIBUTION
- X-Ray photoelectron spectroscopy (XPS)
- Transmission electron microscopy (TEM)
- Xray diffraction (XRD)
- Scanning electron microscopy (SEM)